
 Go to 2002 RSC Annual Report Index
Go to 2002 RSC Annual Report Index
Inorganic Chemistry
Synthetic Organometallic and Coordination Chemistry
Professor Anthony Hill
http://rsc.anu.edu.au/research/hill.php
Our work covers a wide range of topics in coordination and organometallic chemistry, focusing primarily on ligand reactivity and transformations. Particular foci include unsaturated ligands involving metal-carbon, metal-phosphorus and phosphorus-carbon multiple bonding and the interface of transition and main group chemistries. In attempting to understand and ideally control the reactivity of such systems, the nature of the metal centre is of paramount importance and this may be tuned through variations in oxidation state, d-configuration and most importantly the nature of the co-ligands. Accordingly considerable effort is directed towards the synthesis of new co-ligands which themselves do not directly take part in ligand transformations but may moderate these indirectly. Recently this work has focused on two classes of ligands; based either on polythiamacrocycles or poly(methimazolyl)chelates.
Crystallographic characterisation of new compounds has been carried out in collaboration (with Drs A.C. Willis, A.J. Edwards, Professor A.D. Rae)
Polydentate Thioether Ligands
TTDD
Thioethers are typically weak ligands for transition metals, however the coordinative ability may be enhanced by inclusion of sulfur donors within a macrocycle - i.e., sulfur analogues of crown ethers.
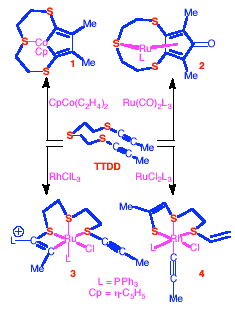 Synthetic routes to such macrocycles are currently under
investigation in this group. Our strategy is based on the
metal-mediated cyclisation of the new a,w-diyne
TTDD. In some reactions of TTDD
with metal complexes, the expected cyclisation products are obtained
by analogy with hydrocarbon a,w-diynes,
e.g., the cobaltacyclopentadiene 1
and the cyclopentadienone complex 2.
In other cases however, the reactivity is diverted as a result of
participation or even fragmentation of the thioether groups, e.g., in
the formation of the metallacycles 3
and 4.(with
L.M. Caldwell, H. Neumann, M. Schultz)
Synthetic routes to such macrocycles are currently under
investigation in this group. Our strategy is based on the
metal-mediated cyclisation of the new a,w-diyne
TTDD. In some reactions of TTDD
with metal complexes, the expected cyclisation products are obtained
by analogy with hydrocarbon a,w-diynes,
e.g., the cobaltacyclopentadiene 1
and the cyclopentadienone complex 2.
In other cases however, the reactivity is diverted as a result of
participation or even fragmentation of the thioether groups, e.g., in
the formation of the metallacycles 3
and 4.(with
L.M. Caldwell, H. Neumann, M. Schultz)
Methimazolylborates and Metallaboratranes
W(CO){H2B(mt)2}2
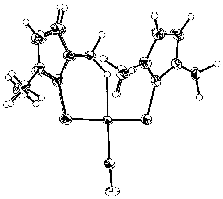 We have isolated a range of cage structures based on a variety of
transition metals ligated by tris(methimazolyl)borates,
HB(mt)3MLn
(mt = methimazolyl). A remarkable feature of these compounds is the
possibility of forming direct transannular dative bonds between the
metal and boron. En route to these novel species, intermediates
involving agostic B-H-M interactions are implicated.
Accordingly, we have turned our attention to the isolation of such
species, by investigating in detail the coordination chemistry of the
simpler bis(methimazolyl)borates, H2B(mt)2. A
clear propensity for agostic interactions has indeed been
established with a number of instances being identified. Amongst
these, the complex W(CO){H2B(mt)2}2
is particularly informative in that it contains two borate ligands,
one with an agostic B-H-W interaction, whilst the other is
simply bidentate. (with E.R. Humphrey, H. Neumann, N.
Tshabang)
We have isolated a range of cage structures based on a variety of
transition metals ligated by tris(methimazolyl)borates,
HB(mt)3MLn
(mt = methimazolyl). A remarkable feature of these compounds is the
possibility of forming direct transannular dative bonds between the
metal and boron. En route to these novel species, intermediates
involving agostic B-H-M interactions are implicated.
Accordingly, we have turned our attention to the isolation of such
species, by investigating in detail the coordination chemistry of the
simpler bis(methimazolyl)borates, H2B(mt)2. A
clear propensity for agostic interactions has indeed been
established with a number of instances being identified. Amongst
these, the complex W(CO){H2B(mt)2}2
is particularly informative in that it contains two borate ligands,
one with an agostic B-H-W interaction, whilst the other is
simply bidentate. (with E.R. Humphrey, H. Neumann, N.
Tshabang)
Transition Metal Propargylidynes: LnM≡C-C≡C-R
Appreciation of the conceptual analogies between the CºC triple bond of alkynes and the MºC triple bond of alkylidynes may provide insights in both the design and interpretation of alkylidyne chemistry. We are currently exploring a special case in which both CºC and MºC bonds are conjugated within the same metallacumulene. Synthetic routes to a range of these have been developed in preparation for a study of their reactivity. (with R. Dewhurst)
Methimazolyl Phosphines and Arsines
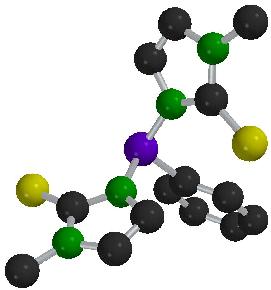
PhP(mt)2
In an extension of our work on methimazolylborates, we have explored the synthesis of phosphine and arsine based ligands which bear methimazolyl substituents. These offer both pnicogen and thione groups for coordination a metal centre. The syntheses of these new ligands is based on the reactions of N-trimethylsilylmethimazole with the appropriate haloarsine or phosphine. This approach has been applied to the synthesis of a range of phosphines and arsines, including the hexedentate derivative. (mt)2PC2H4P(mt)2. Furthermore, our recent isolation of the corresponding silylated selone provides access to selenium analogues (with R. Dewhurst, E.R. Humphrey, and J.D. Woollins [U. St Andrews])